By Dr. Mercola
When most people think of pollution, they think of the outdoors—garbage-choked streams or industrial waste. But you probably spend a large portion of your time indoors—as much as 80 to 90 percent of your life. You work, study, eat, drink and sleep in enclosed environments where air circulation may be restricted.
The typical American home contains 3-10 GALLONS of toxic materials—everything
from glass and bathroom cleaners to garden pesticides and fertilizers.
Health
effects of ingredients in common household products include:
·
Respiratory problems
·
Eye irritation
·
Cancer
·
Disruption of the endocrine system
As a result of cleaners and
other toxic household products, the Environmental Protection Agency
(EPA) reports that the air inside the typical home is 2-5 times more polluted
than the air immediately outside—and in extreme cases, 100 times more
contaminated.
In one New York medical center, reports of burns, rashes,
dizziness and scratchy throats among hospital employees plummeted after the staff
switched over to less toxic cleaning products. The number of missed
work days due to cleaning product injuries declined from 54 in 2004 to zero in
2009
Getting Down on Dirty
Detergents
The average family washes approximately 80 pounds of laundry per
week—or 35 billion loads
of laundry per year! This means that 17.5 billion
cups of laundry detergent are being used every year in the U.S. alone. Not only
can you come in contact with caustic chemicals via your clothing, from having
been laundered in them, but you can breathe them into your lungs once they
become airborne in the process of doing your laundry.
The
detergent you're using may contain a cocktail of potent cancer-causing chemicals,
some of which the manufacturer doesn't even have to list on the label. This
loophole reduces the odds that you'll ever discover what's in there.
Four of the worst offenders are:
1.
Sodium
lauryl sulfate (SLS)/sodium laureth sulfate (SLES).
2.
1, 4-dioxane.
3.
NPE
(nonylphenol ethoxylate).
4.
Phosphates.
Not only are these chemicals
potentially damaging to your health, but they are also contaminating waterways
and harming the environment.
1. Sodium Lauryl Sulfate (SLS),
Sodium Laureth Sulfate (SLES), and Ammonium Laurel Sulfate (ALS)
Sodium lauryl sulfate is a surfactant, detergent and emulsifier
used in thousands of industrial cleaners and cosmetic products. It is present
in nearly all shampoos, scalp treatments, hair color and bleaching agents,
toothpastes, body washes and cleansers, make-up foundations, liquid hand soaps,
and laundry detergents.
Although SLS originates from coconuts, the chemical is anything but natural.
SLS is mixed with sulfur trioxide or chlorosulfuric acid and then
neutralized with aqueous sodium hydroxide (lye). SLS is the sodium salt of
lauryl sulfate and is classified by the Environmental Working Group (EWG)
Cosmetics Database as a "denaturant, surfactant cleansing agent,
emulsifier and foamer," rated "moderate hazard."
Similar to sodium lauryl sulfate
(SLS) is sodium laureth sulfate (short for
sodium lauryl ether sulfate, or SLES), a yellow detergent with higher foaming
ability. SLES is considered to be slightly less irritating than SLS. SLS goes
by other names, including:
·
Sodium
dodecyl sulfate
·
Sulfuric
acid, monododecyl ester, sodium salt
·
Sodium
salt sulfuric acid
·
Monododecyl
ester sodium salt sulfuric acid
·
A13-00356
·
Akyposal
SDS
·
Aquarex
ME
·
Aquarex
methyl
Ammonium lauryl sulfate (ALS) is another variation commonly put
into cosmetics and cleansers to make them foam. ALS is similar to SLS, showing
similar risks.
Sixteen Thousand Studies Document the Hazards of SLS
According to the Environmental
Working Group's Skin Deep: Cosmetic Safety Reviews, research studies
on SLS have shown links to:
·
Irritation
of the skin and eyes
·
Organ
toxicity
·
Developmental/reproductive
toxicity
·
Neurotoxicity,
endocrine disruption, ecotoxicology, and biochemical or cellular changes
·
Possible
mutations and cancer
If you visit the SLS page on
EWG's website, you will see a very long list of health concerns and associated
research studies. In fact, you will also see mention of nearly 16,000 studies
in the PubMed science library (as well as their link to that list) about the
toxicity of this chemical.
A number of studies report SLS being damaging to oral mucosa and
skin. This is not at all surprising since SLS is actuallyused as a skin irritant during studies where
medical treatments for skin irritation require first using an intentionally
irritating agent. A study appearing
in Exogenous Dermatology confirmed SLS to be a very
"corrosive irritant" to the skin—irritation which persisted in
research subjects for 3 weeks. SLS exerts its damage by stripping your skin of
protective oils and moisture.
SLS has also been linked to nitrosamines. Nitrosamines are
potent carcinogens that cause your body to absorb nitrates,
also known to be carcinogenic.
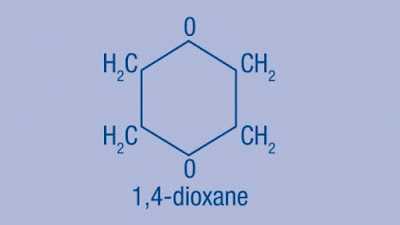
2. Two-Thirds of Laundry Detergents
Contain 1,4 Dioxane
David Steinman, an environmental health consumer advocate with the
Green Patriot Working Group (GPWG) and former representative at the National
Academy of Sciences, has been on a mission since 2007 to organize product
testing for the petrochemical 1,4-dioxane in your personal care and household
cleaning products. He forged a partnership between his organization and the
Organic Consumers Association (OCA) to get the dirt on dioxane-laden products.
In 2008, the focus was personal care products, and 2010 has
brought the spotlight to laundry detergents. In 2008, the findings were
shocking.
Many popular brands of shampoos, body washes, lotions, and even
baby products—as well as many "natural" and "organic"
brands—were found to
contain 1,4-dioxane.
Levels of contamination were so high that many companies have come
under legal attack for poisoning consumers. Unfortunately, this phase of
testing proved no lesser threat. About two-thirds of the laundry detergents tested contained 1,4-dioxane.
Results suggest it's time for these companies to clean up their acts.
It is reassuring, however, that all brands with the USDA
organic certification were found to be dioxane-free.
At a press conference in Anaheim, California, on March 12, 2010,
Steinman shared the test results from 20 laundry detergents—13 conventional
brands and 7 "natural" brands. As you would expect, the natural brands
fared better.
The Organic Consumers Association and Green Patriot Working Group
have put together a handy printable
guide for Personal Care and Cleaning Products that includes
everything from dish soap to hand soap to deodorant, and everything in between.
Why You Should be Concerned About
1,4-Dioxane
Don't confuse 1,4-dioxane with dioxin— they
are completely different compounds. Dioxin is
not manufactured commercially but is a byproduct of combustion. For example
forest fires and the burning of garbage, produces a family of 17 different
compounds of varying toxicities. Dioxane (also
called 1,4-dioxane) is a byproduct of an industrial process used to make
cleaning ingredients, and this is what can contaminate your personal care and
cleaning products.
How does 1,4-dioxane get into your products? It's not added intentionally. As I mentioned earlier, it is a
by-product of SLS, which is an extremely common ingredient in detergents.
According to the "1,4-Dioxane
Product Safety Watch" website, dioxane
is a byproduct of ethoxylation, "a cheap shortcut process
companies use to provide mildness to harsh cleaning ingredients."
Ethoxylation involves combining low-sudsing ingredients with ethylene oxide (which
is a known human carcinogen) to produce softer detergents that produce more
suds. The result is diethylene oxide, or 1,4-dioxane, or simply dioxane.
Since it is a byproduct rather than ingredient, it doesn't have to
be listed on product labels. But you really DON'T want to have your skin coming
into contact with this stuff, byproduct or not. 1,4-dioxane is considered by
the State of California to cause cancer
and has been found to be potentially toxic to your brain and central nervous
system, kidneys, liver and respiratory system, according to the CDC. According to
the Organic
Consumers Association's 1,4-Dioxane Facts Sheet:
·
The
cumulative effects of 1,4-dioxane exposure, even at very low levels (a few
parts per billion) resulted in laboratory animals developing cancer.
·
1,4-dioxane
is readily absorbed through the lungs, skin and gastrointestinal tract of
mammals.
·
The U.S.
federal regulation systems consider dioxane's potency to be equivalent to or
greater than many pesticides considered dangerous to humans.
·
Cosmetics
(and detergents, presumably) contaminated with 1,4-dioxane may also have traces
of other contaminants, including formaldehyde, nitrosamines, and phthalates.
·
There are
many inexpensive and effective alternatives to ethoxylation in the
manufacturing of your personal care and cleaning products.
The National Institute of Health (NIH) substance profile
sheet confirms that 1,4-dioxane is "reasonably expected to
be a human carcinogen" based on the research to date, and even trace
amounts bring cause for concern.
3. NPE (Nonylphenol Ethoxylate)—the
"Gender Bender"
Like SLS and SLES, NPE is an inexpensive nonionic surfactant
frequently used in laundry detergents. NPE is an
endocrine disruptor and estrogen mimicker that can potentially
cause hormonal problems, or even cancer. When you absorb NPE, your body can't
tell the difference between NPE and estrogen.
Organisms exposed to NPE show kidney and liver damage, decreased
testicular growth and sperm count, disrupted growth and metabolism, and
increased mortality.
When rainbow trout are exposed to NPEs, they become part male and
part female! According to the Sierra Club, who recently petitioned the EPA to
regulate NPE, roughly 270 million
pounds of NPE are used in the United States each year—and the
majority of this ends up being rinsed down your drain. A U.S. Geological Survey
study found metabolites of NPEs in more than 61 percent of tested streams in
the U.S. (reported by Sierra Club).
According to a Sierra Club
paper, researchers now believe that:
"NPE pollution is likely to be at least partly responsible
for a variety of odd gender bending phenomenon now being seen in aquatic
species. And while human effects remain unknown, scientists believe it may be
affecting people, too."
NPEs have been banned already in Canada and Europe. Even Wal-Mart
has listed NPEs as one of three chemicals they're asking suppliers to phase
out.
Even the most sophisticated water treatment plants are unable to
remove NPEs and their toxic metabolites. In fact, according to the Sierra Club
report, sewage processing can make NPE metabolites more
toxic, more estrogenic, and more persistent than NPE itself.
Look for evidence of NPE on your laundry detergent label—or
declaration that it's not in there. Some detergents contain NPE alternatives
such as alcohol ethoxylate, which the Sierra Club suggests is less toxic and
can break down naturally. Another enormous threat to your water supply is
phosphates.
4. Phosphates and the Choking of
Aquatic Life
Phosphates are the main cleaning ingredient in many detergents and
household cleaners because they break down dirt particles and remove stains by
softening the water and allowing suds to form, which enhances the cleaning power
of the detergent. Some dishwasher tabs are more than 30 percent phosphates!
However, there are human health problems as well as major
environmental hazards associated with phosphates. Phosphate residues on items
that have been cleaned with phosphate-containing detergents have been known to
cause nausea, diarrhea
and skin irritations.
The largest concern with phosphates, however, is the environmental
hazards they are creating.
Phosphates are difficult to remove from wastewater and often end
up in rivers and lakes, where they increase algae growth, choking off waterways
and suffocating salmon and other aquatic life, literally starving them of
oxygen. Phosphates act like a "fertilizer" in waterways. When the
overabundant algae die, they release toxins that deplete the waterways of oxygen.
Phosphates remain active even after wastewater treatment.
Pretty Scary Laundry List
Besides surfactants and phosphates, the average detergent has a
long list of other synthetic chemical ingredients—and most are not good for you
or the Earth. Anything in those products can potentially be absorbed through
your skin or breathed in through your nose, as well as passed down the drain to
our waterways.
Typical chemicals include:
- Linear alkyl sodium sulfonates (LAS), a.k.a.
anionic surfactants
- Petroleum distillates (a.k.a. naphthas), which
have been linked to cancer
- Phenols, which can cause toxicity throughout
the entire body
- Optical brighteners, which cause bacterial
mutations and allergic reactions, and can be toxic to fish
- Sodium hypochlorite (bleach)
- EDTA (ethylene-diamino-tetra-acetate)
- Artificial frag
Dangerous substances like sodium
laurel sulfate (SLS), ammonia, household bleach, nonylphenol ethoxylate,
phenols, and phosphates can instigate a host of other significant health
problems once they penetrate your skin.
Recommendations
A. Flush Your Body Clean of these Toxins.
Environmental
toxins are responsible for many cancers, neurological diseases, heart disease,
you name it. Our bodies do have a built-in detox function to deal with these
dangers, but those systems are constantly overloaded! Detoxing assists and
improves what our bodies are trying to do naturally.
Cleaning your body from toxins is
a necessity for optimum health. You may love the smell of your favourite
detergent, but there’s no way it’s worth side effects like corneal damage and
pneumonia, or worse yet, cancer. Exposure to these toxins is lethal. You have
to flush them out with a natural detox juice.
You can prepare your own detox
recipe at your home and buy a natural detox from the market. There are a lot
natural detox juice on the market but I personally recommend organifi.
Those toxins need to exit the body.
When you detox the body you free up your organs to function the way they should.
B. Go natural
Clearly, the solution to this
serious dilemma is not to stop laundering your clothes altogether. Lee Euler,
editor of Cancer Defeated, has some much more realistic tips on how to minimize
your risk:
1.
Wear your clothes longer before
laundering them— 2 or 3 times, especially during cooler seasons when they’re
barely soiled after one wearing.
2.
Use 100% non-toxic, biodegradable
laundry products.
3.
Use phosphate-free products.
4.
Seek out plant-based enzymes and
food-based detergents.
5.
Say no to bleach, dyes,
fragrances, optical brighteners, and masking agents.
6.
Reduce the amount of detergent per
load. Remember, the manufacturer’s goal is to sell as much product as possible.
You might find your clothes get just as clean with ¼ cup of detergent as with ½
cup. Plus, you’ll save money.
References
3,,,Mercola, J. “Are You Poisoning Your
Household With this Chore?."
Retrieved January 03, 2016, from
Annmarie Skin Care, 7 Toxic Chemicals Found in
“Clean” Laundry.
accessed january 03, 2017 from